Aritinercept
Aritinercept Is a Dual BAFF/APRIL Inhibitor for the Potential Treatment of Autoimmune Diseases
Aritinercept contains a B cell maturation antigen (BCMA)-engineered extracellular binding domain optimized for superior affinity to B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL) (other dual BAFF/APRIL inhibitors use transmembrane activator and CAML interactor [TACI]-engineered extracellular binding domain). BCMA has a stronger natural affinity for APRIL than TACI. a
Aritinercept contains an immunoglobulin (Ig) G4 fragment crystallizable region (Fc) domain with no appreciable effector function (other dual BAFF/APRIL inhibitors use IgG1 Fc domain). IgG4 is considered the least inflammatory across the IgG subclasses, in part because it poorly activates the complement system. b
a Mathur et al., J Clin Med 2023
b Oskam et al., Front Immun 2023

Role of BAFF and APRIL
BAFF and APRIL are important cytokines that regulate B cell survival and differentiation, whose targets are expressed on B cells at different stages of B cell development. a Targeting both BAFF and APRIL depletes a broader set of B cells, including plasma cells, than targeting a single cytokine. Aritinercept may prevent the activation of autoreactive B cells and reduce their numbers and associated immunoglobulins (antibodies) in the body, thereby reducing important drivers of B cell-mediated autoimmune diseases.

a Mathur et al., J Clin Med 2023
b Schrezenmeier et al., J Am Soc Nephrol 2018
Aritinercept Is a High Affinity Dual BAFF/APRIL Inhibitor
Aritinercept has high binding affinity for both BAFF and APRIL as compared to other dual BAFF/APRIL inhibitors.

a Data on file
Aritinercept Potently Inhibits BAFF- and APRIL-Mediated B Cell Proliferation
Aritinercept potently inhibits both BAFF- and APRIL-mediated B cell proliferation as compared to other dual BAFF/APRIL inhibitors.

a Data on file
Aritinercept Single Ascending Dose (SAD) Study: Design
The study investigated aritinercept doses of 5 mg, 25 mg, 75 mg, 150 mg, 225 mg and 300 mg and placebo, administered by subcutaneous injection, in 61 healthy subjects.
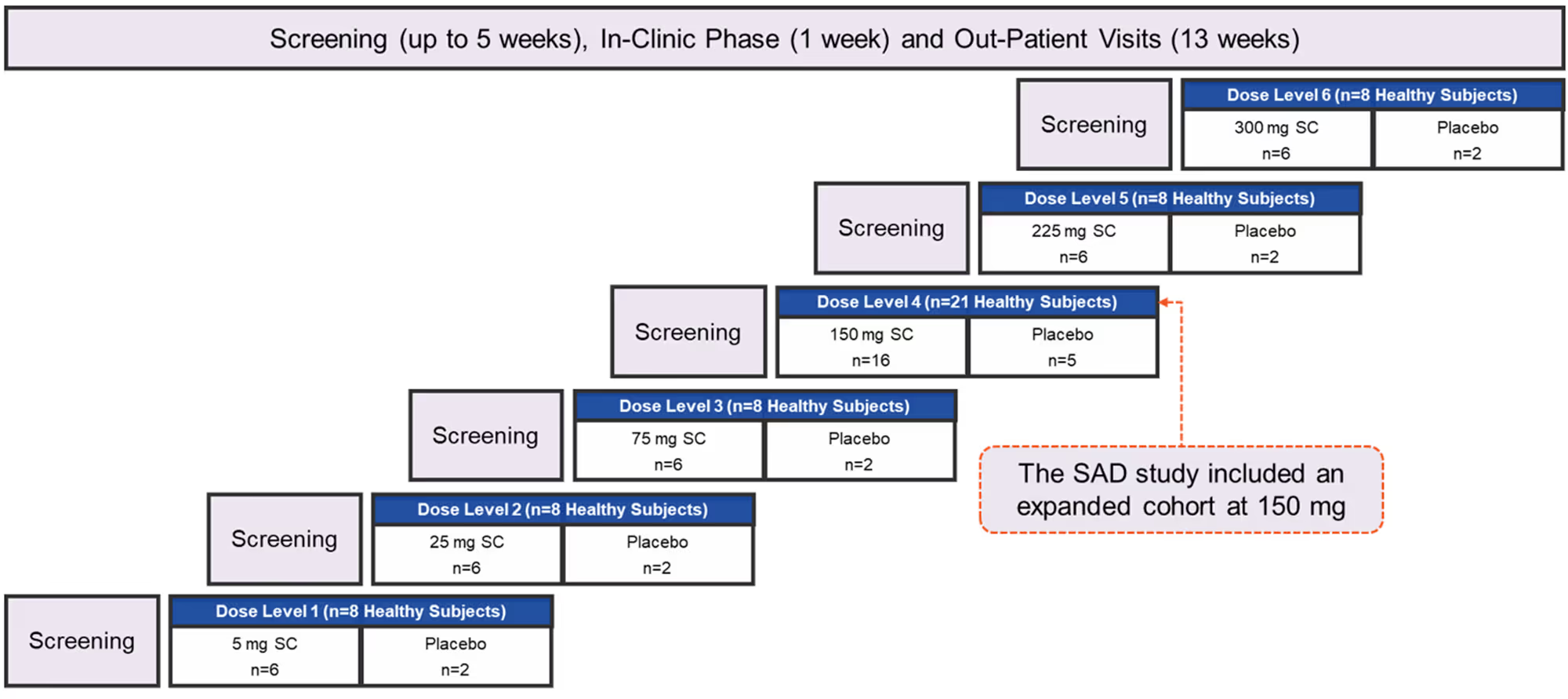
SC=subcutaneous
Aritinercept SAD Study: Safety Summary
Aritinercept was well tolerated at all dose levels tested. There were no treatment-related Grade ≥3 adverse events (AEs), there were no treatment‑related serious adverse events (SAEs) and there were no discontinuations due to treatment-related AEs. There was 1 Grade ≥3 AE and 1 SAE (same event) of concussion due to motor vehicle accident reported as not treatment related. AEs that occurred in more than one subject included injection site reactions (24% aritinercept, 13% placebo), headache (11% aritinercept, 7% placebo), upper respiratory tract infection (7% aritinercept, 0% placebo) and back pain (4% aritinercept, 0% placebo). All injection site reactions were Grade 1.
Anti‑drug antibodies (ADAs) were detected in the majority of subjects at dose levels of 25 mg and higher. The presence of ADAs was not associated with any changes in safety, pharmacokinetic or pharmacodynamic parameters.
Aritinercept SAD Study: Single Doses of Aritinercept Led to Robust and Long-Lasting Reductions in Immunoglobulins in Humans
Single doses of aritinercept led to robust and long-lasting reductions in immunoglobulins (antibodies). Specifically, mean reductions from baseline to Day 28 of up to 48%, 55% and 20% were observed for IgA, IgM and IgG, respectively. Pharmacodynamic effects are supportive of once-monthly dosing.

Effect of a Single Dose of BAFF/APRIL Inhibitors on IgA, IgM and IgG
Below are comparative results of the reduction in immunoglobulins IgA, IgM and IgG, respectively, between aritinercept and other BAFF/APRIL inhibitors.
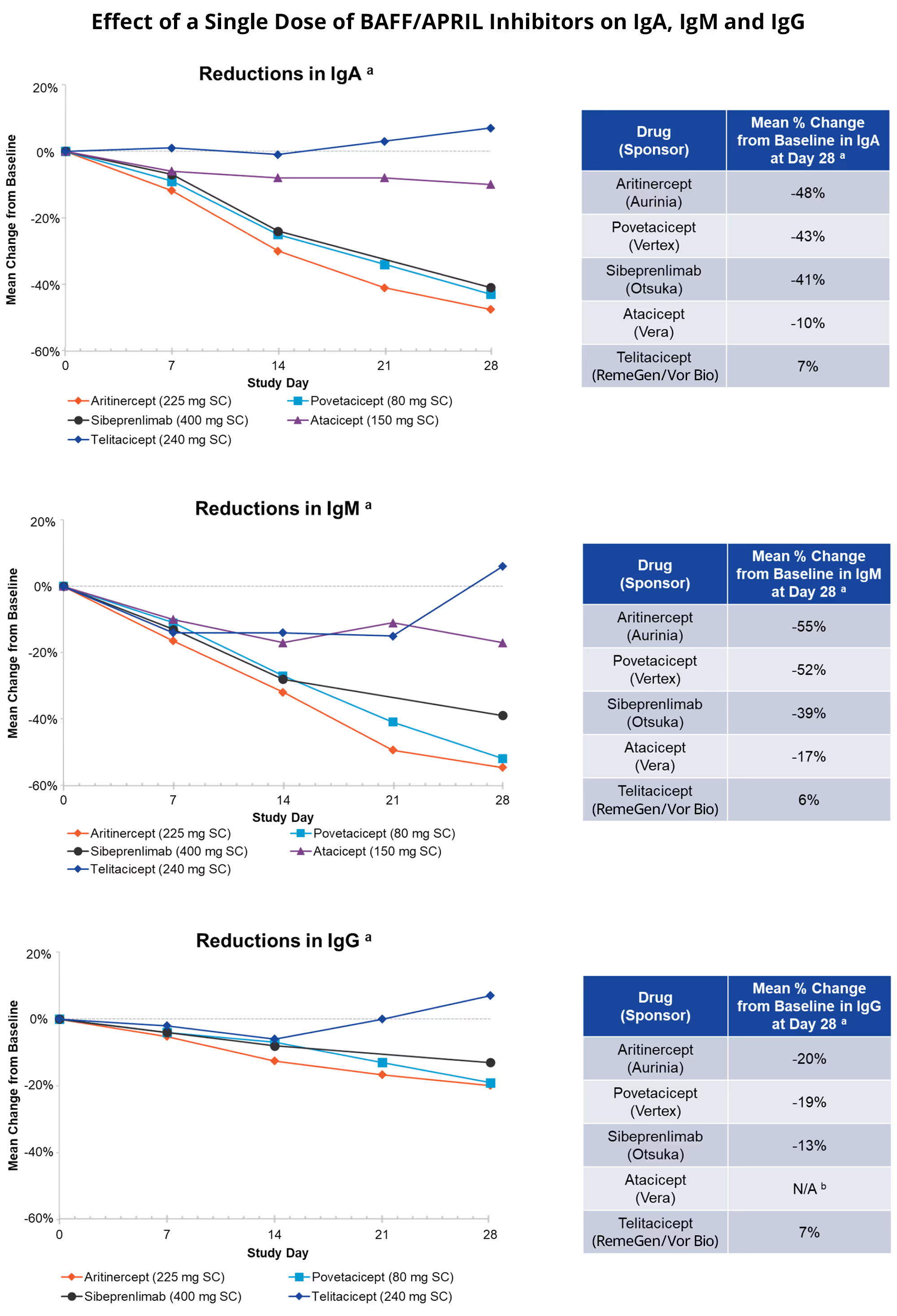
a The figures and tables above represent cross-trial comparisons of SAD studies. No head-to-head clinical studies have been conducted. Adapted from Davies et al., Clin Trans Sci 2024 (povetacicept); Zhang et al., ClinPharm Drug Dev 2023 (sibeprenlimab); Willen et al., Eur J Drug Metab Ph 2020 (atacicept); Xie et al., Clin Pharm Drug Dev 2022 (telitacicept). Dose levels for povetacicept, sibeprenlimab, atacicept and telitacicept represent dose levels selected by respective sponsors for Phase 3 development.
b There was no apparent reduction in serum IgG levels following single-dose atacicept at any of the tested doses
Summary and Next Steps
Aritinercept was well tolerated at all dose levels tested. Single doses of aritinercept led to robust and long-lasting reductions in immunoglobulins supportive of once-monthly dosing. Aurinia has initiated a clinical study of aritinercept in one autoimmune disease and plans to initiate a clinical study in an additional autoimmune disease in the first half of 2026.
