Investors
Corporate Overview
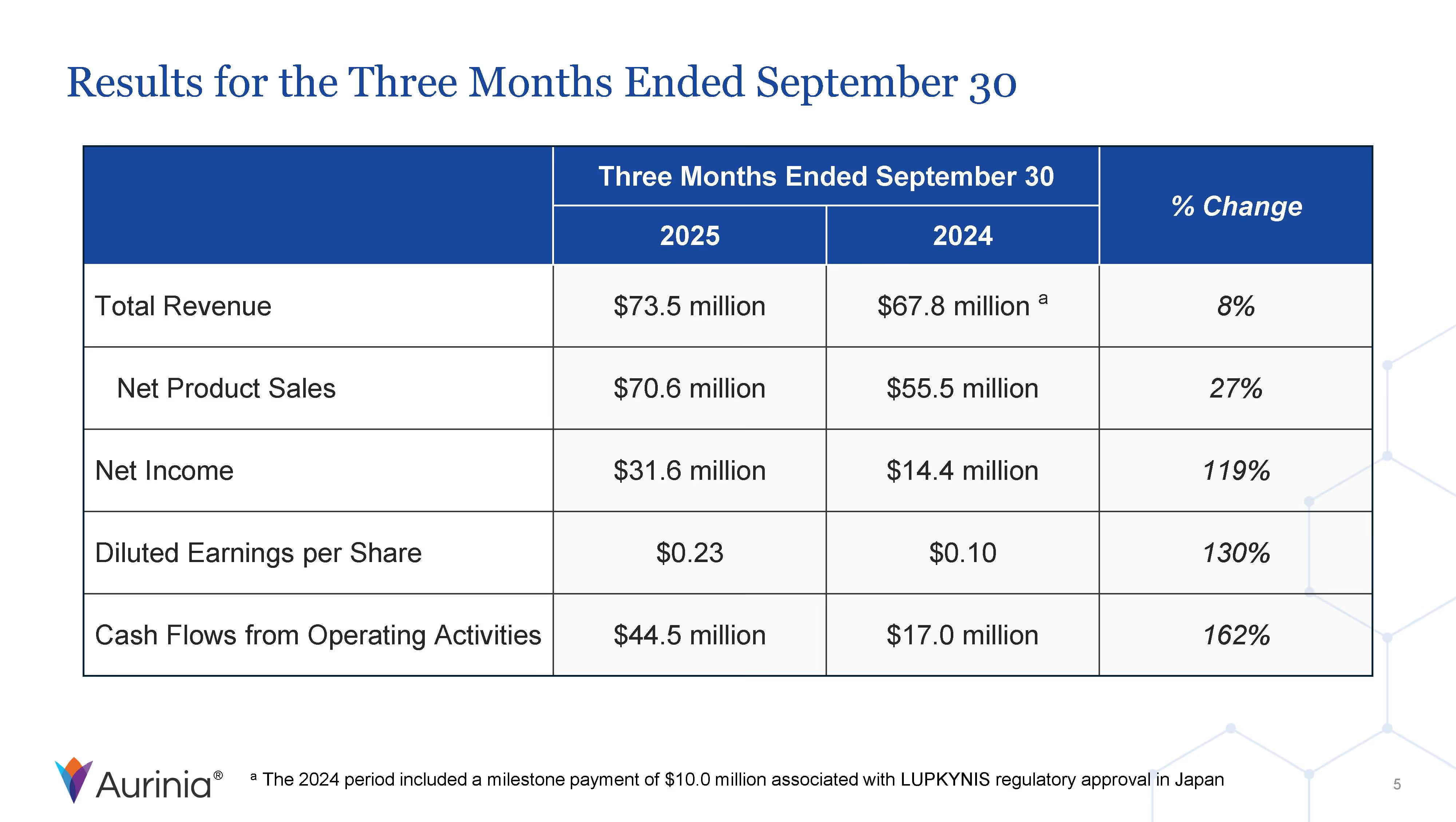
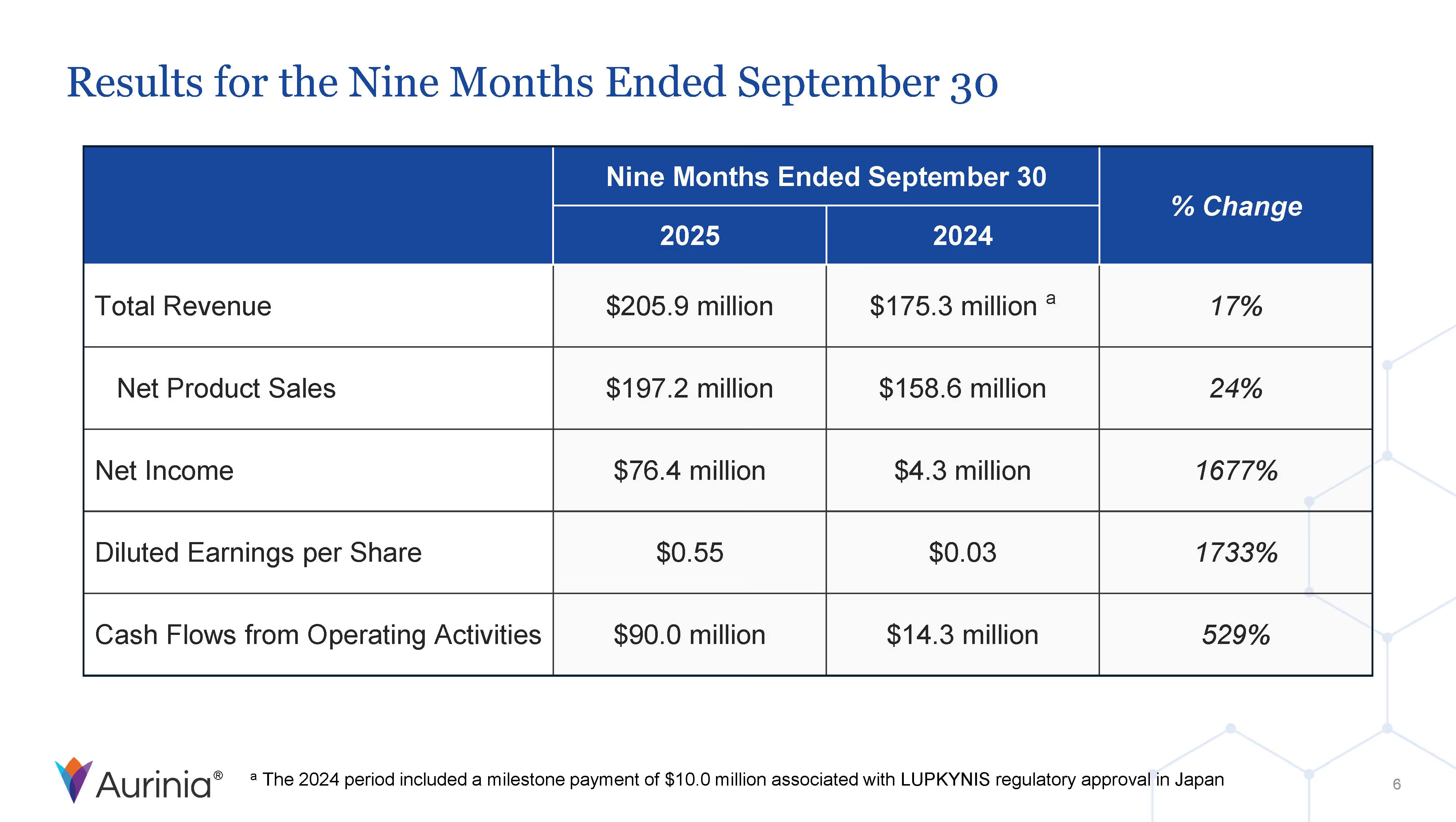
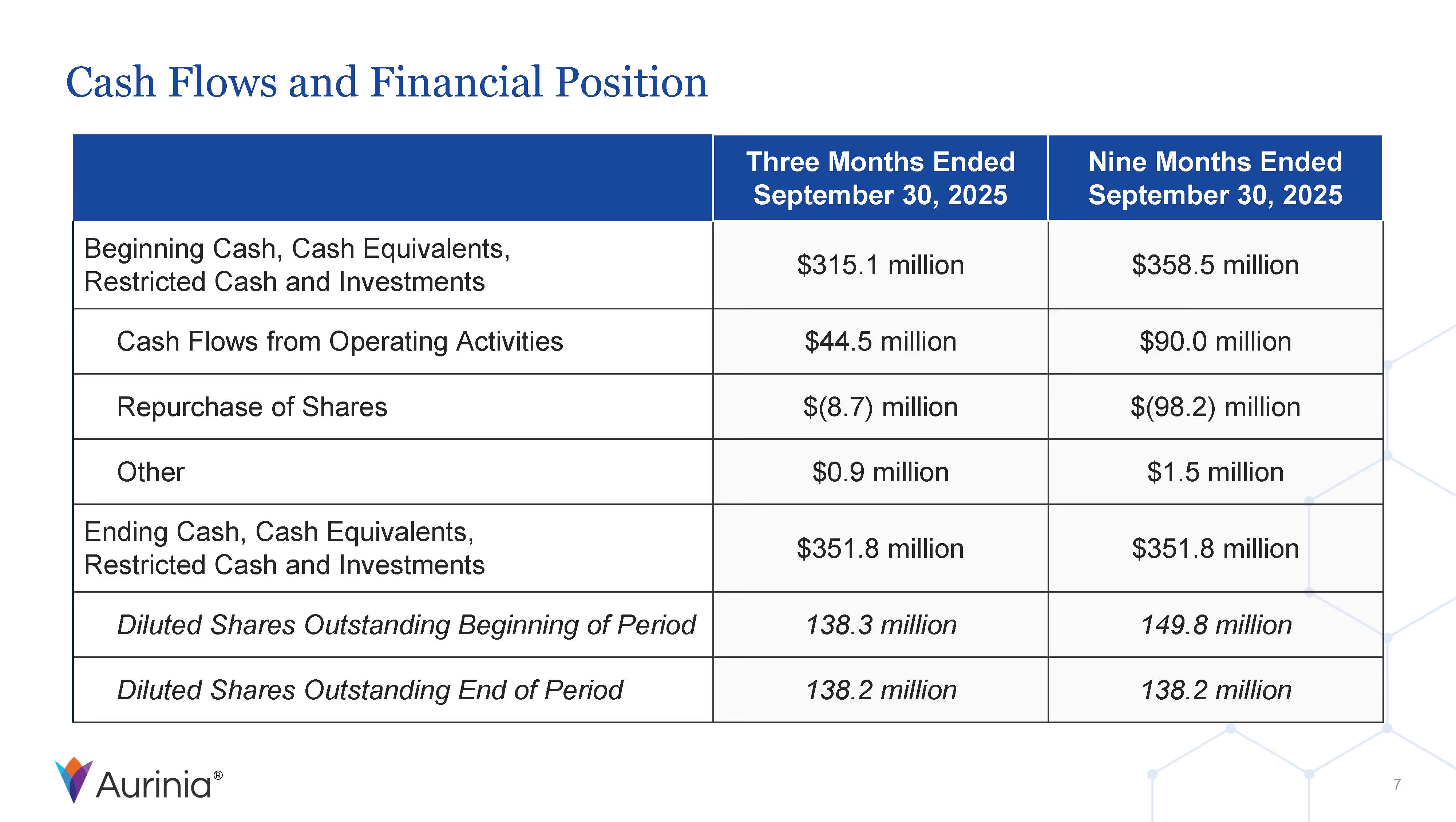
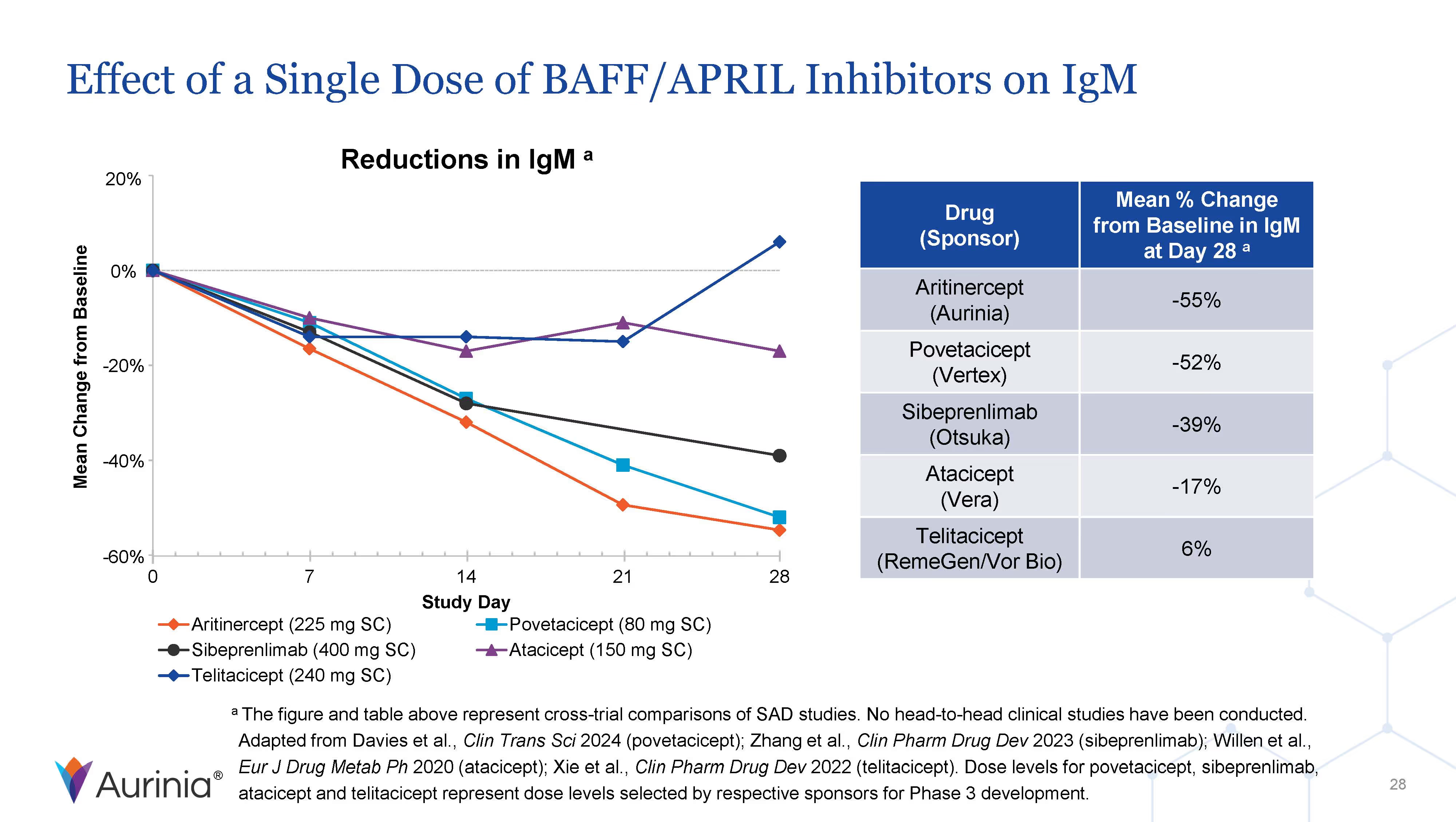
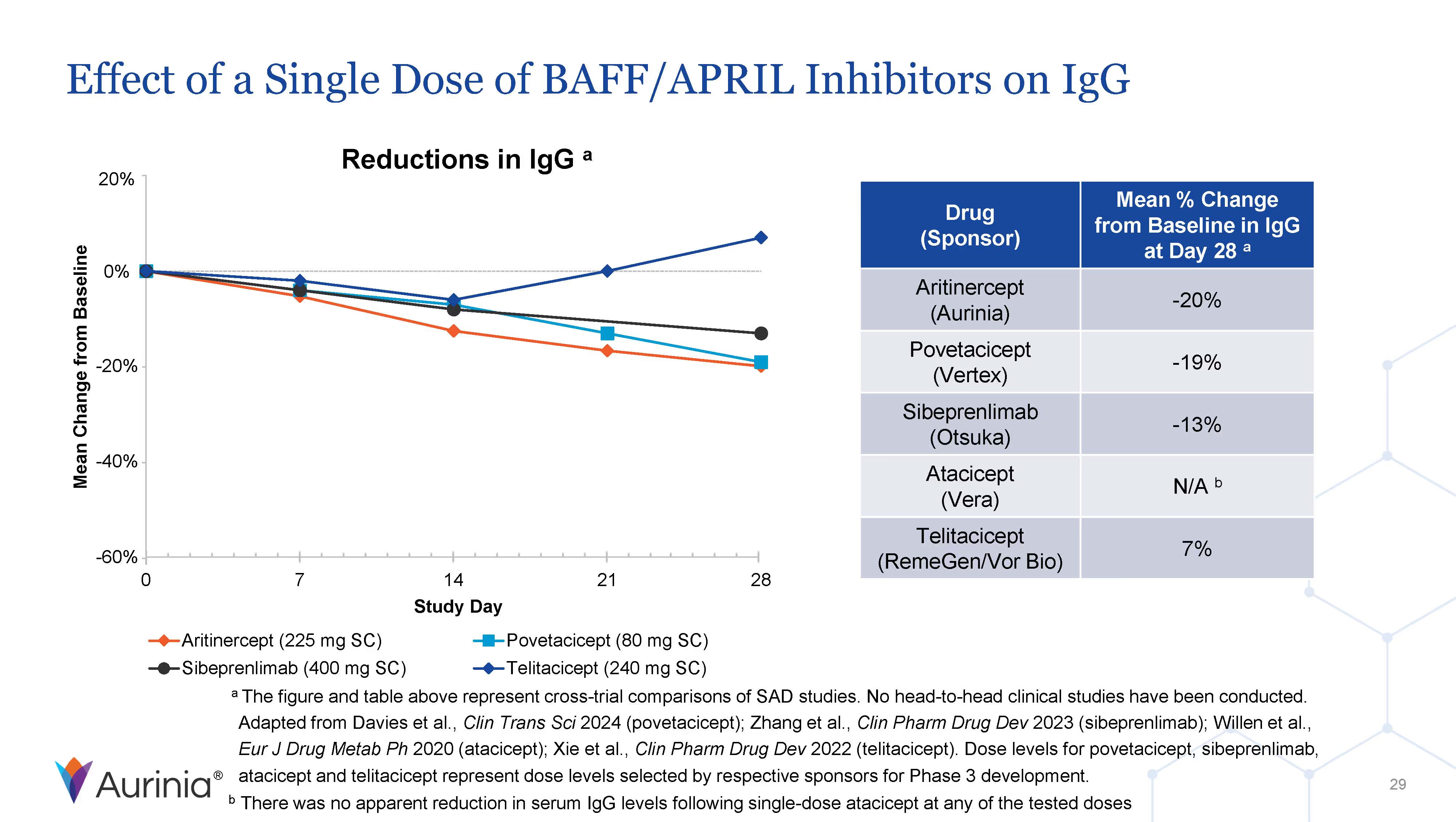
Aurinia Pharmaceuticals Inc. is a biopharmaceutical company focused on delivering therapies to people living with autoimmune diseases with high unmet medical needs. In January 2021, the Company introduced LUPKYNIS (voclosporin), the first FDA-approved oral therapy for the treatment of adult patients with active lupus nephritis. Aurinia is also developing aritinercept, a dual inhibitor of B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL) for the potential treatment of autoimmune diseases.
Email Alerts
Stock Information
NASDAQ Global Market: AUPH
+0.08
+0.54%
Volume
Upcoming Events
Past Events
National Kidney Foundation (NKF) Spring Clinical Meeting 2024
Leerink Partners Global Biopharma Conference
First Quarter 2024 Financial Results Conference Call
2024 Bloom Burton & Co. Healthcare Investor Conference
TD Cowen’s 44th Annual Health Care Conference
SEC Filings
Governance
IR Contact
General inquiries can be sent to ir@auriniapharma.com