Investors
Corporate Overview
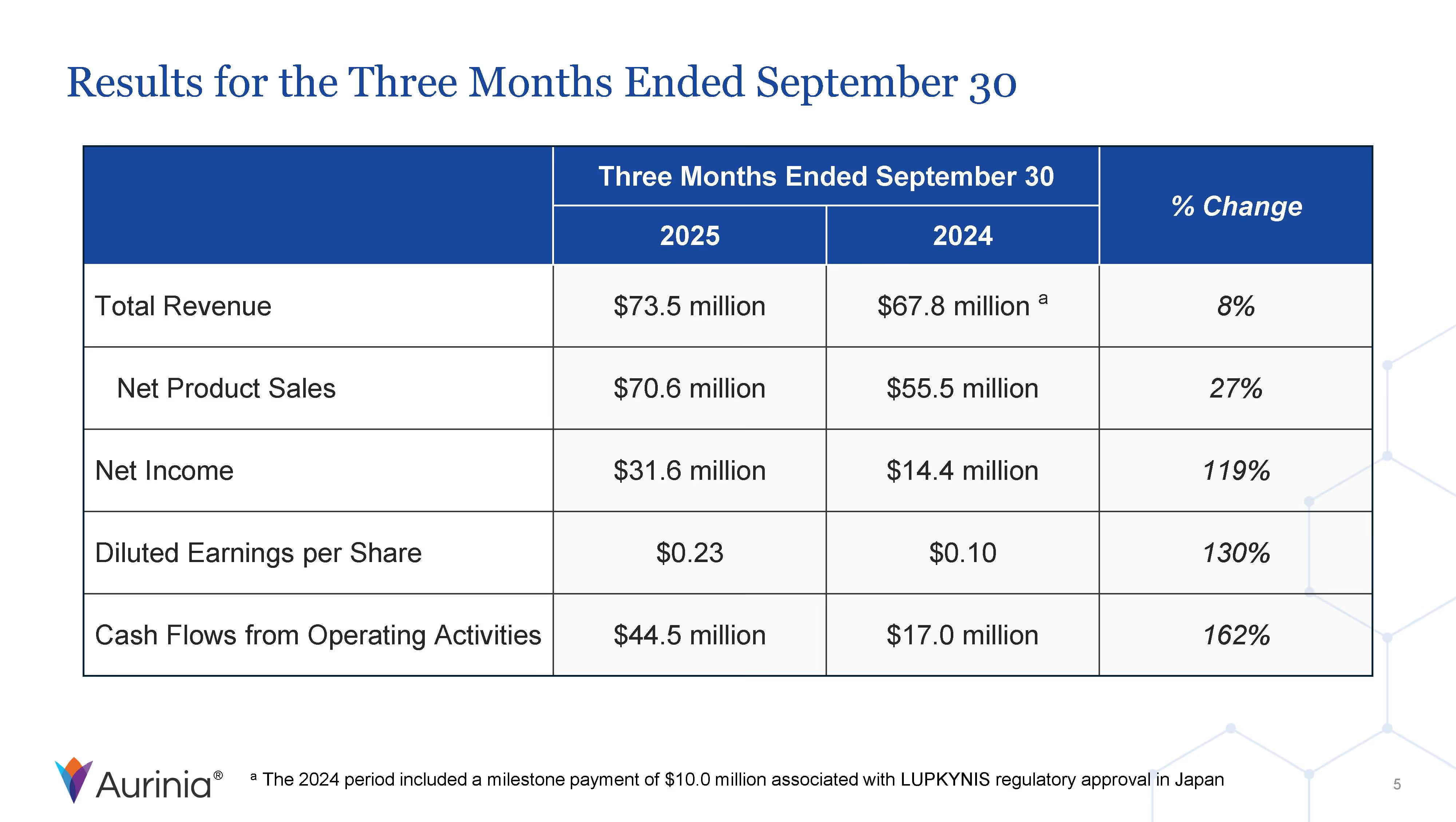
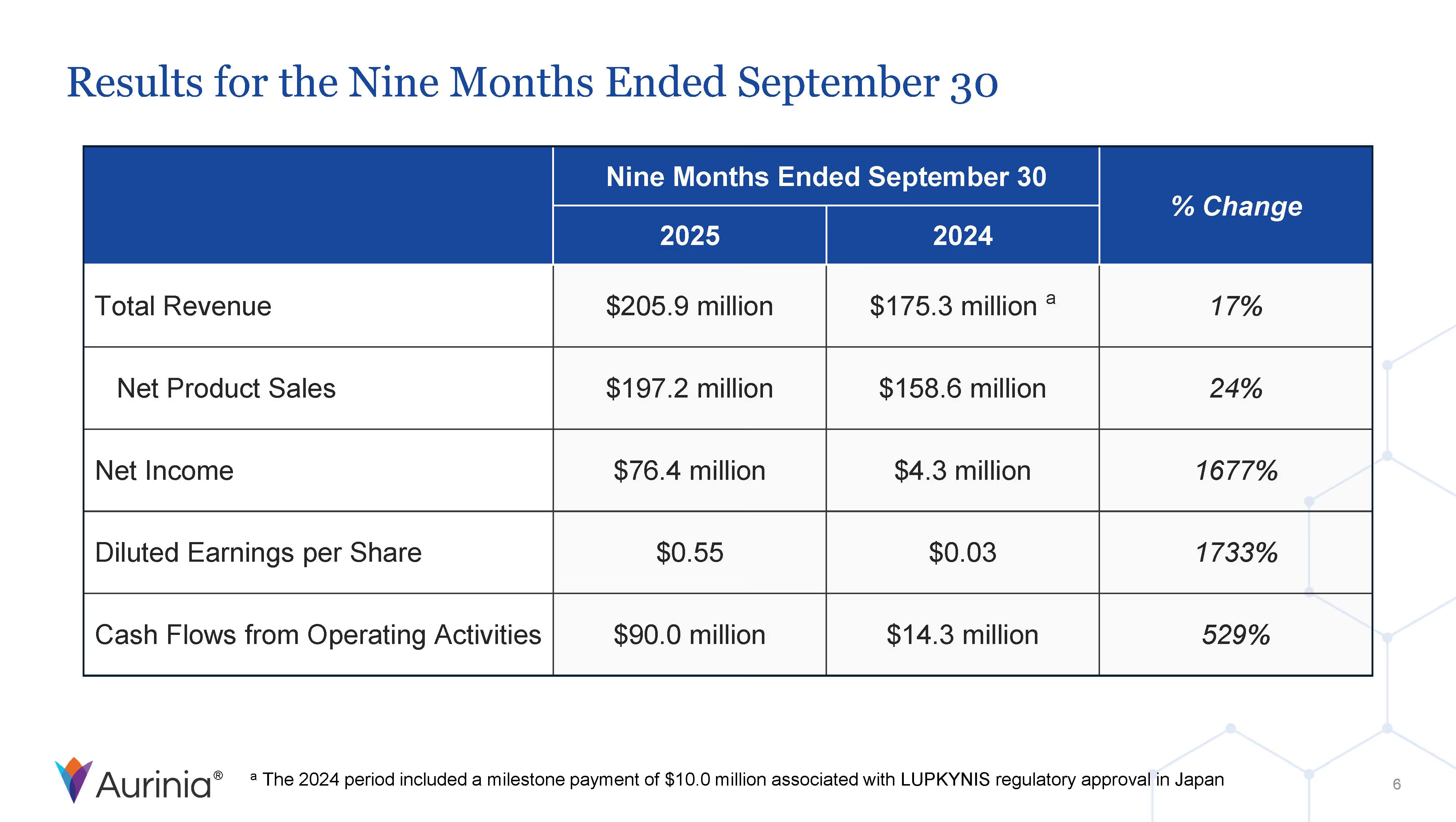
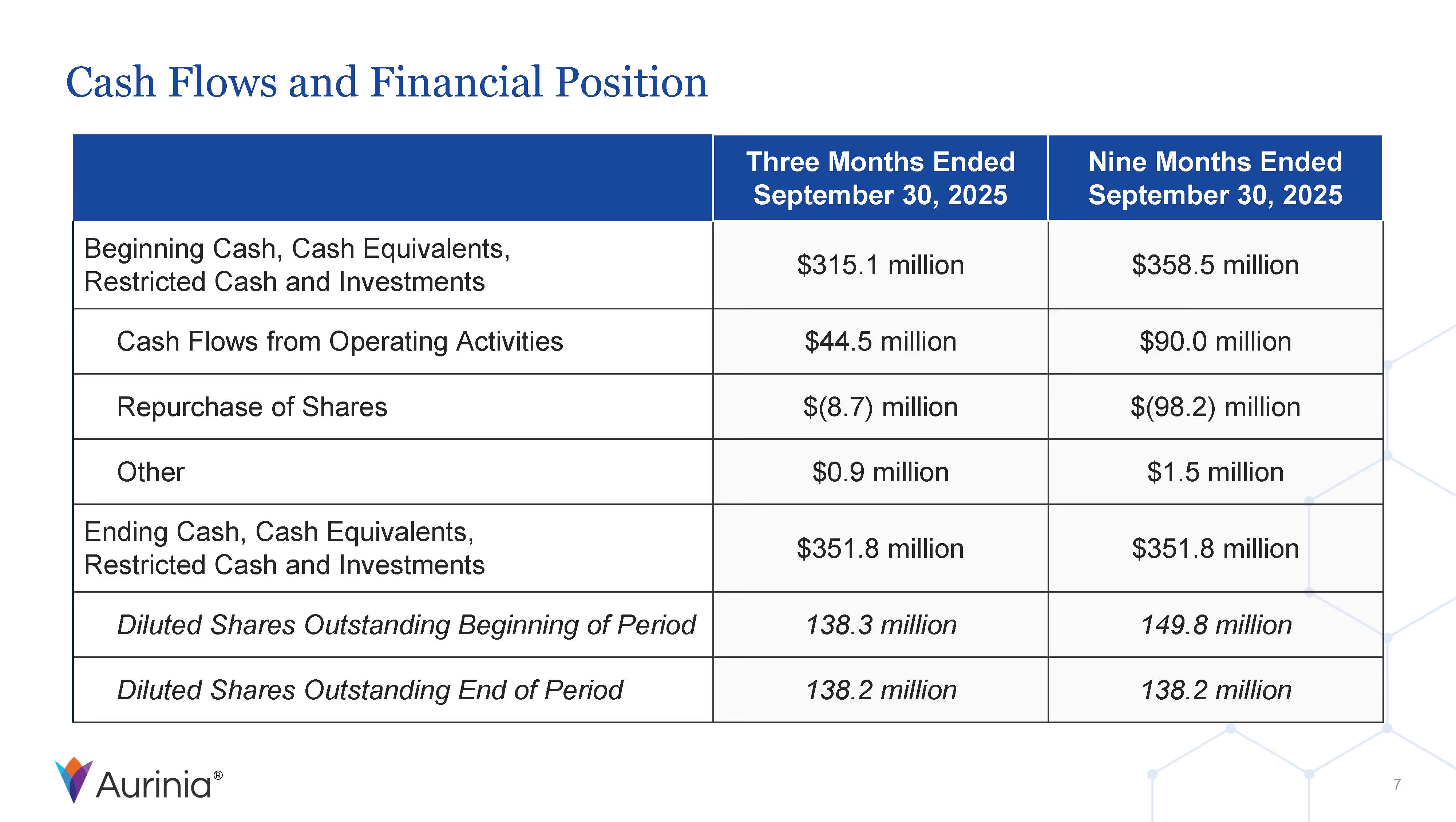
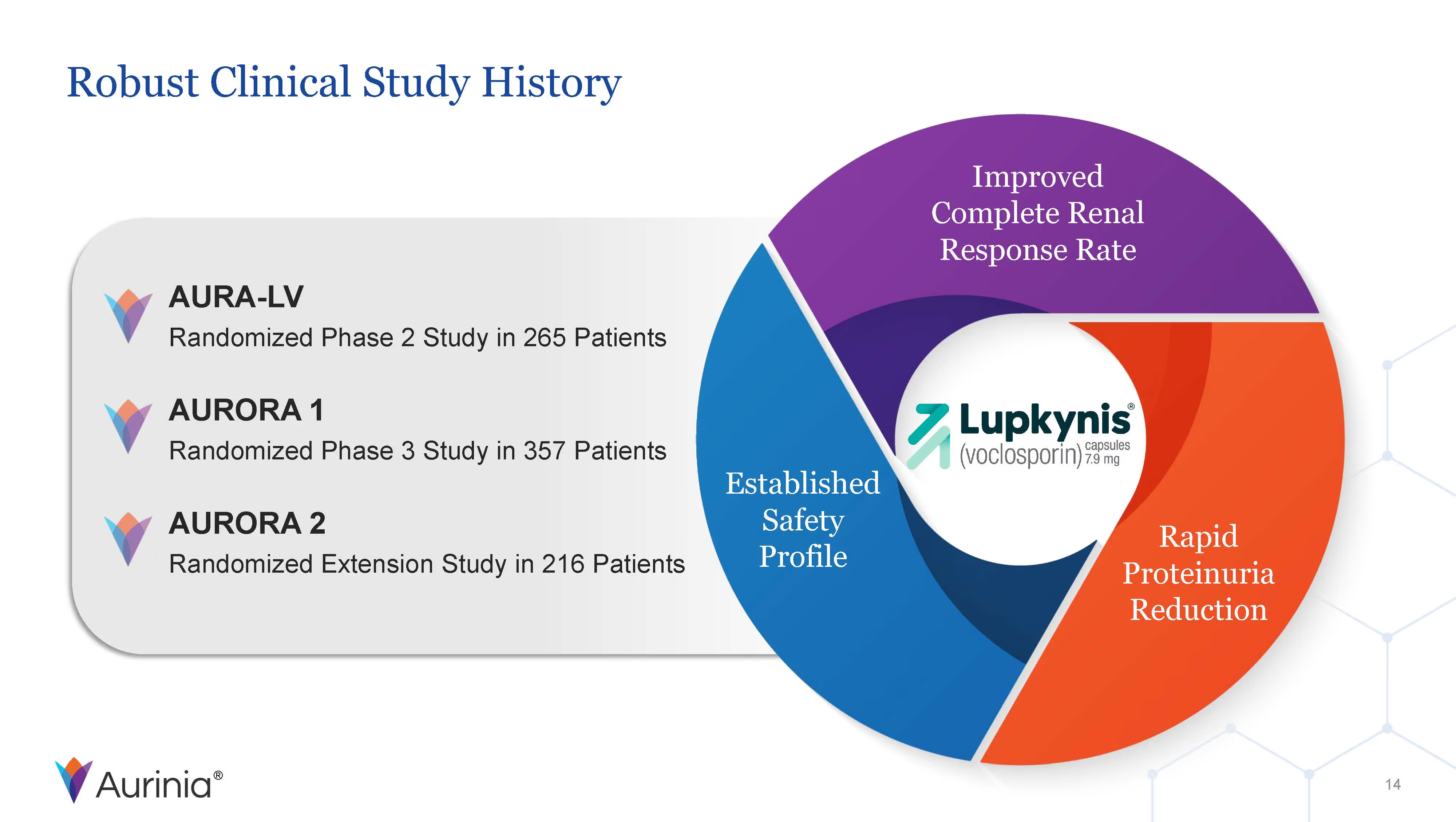
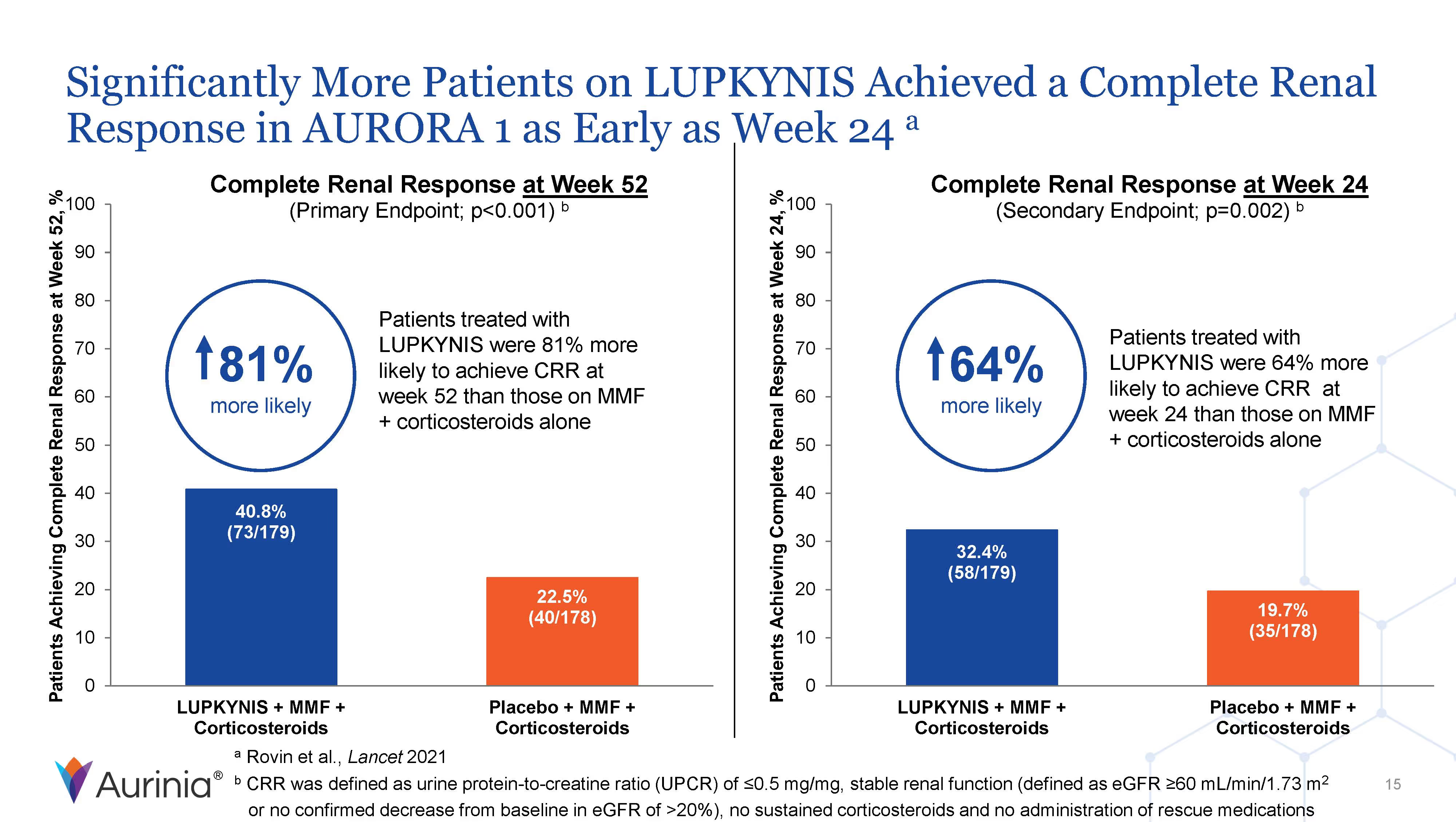
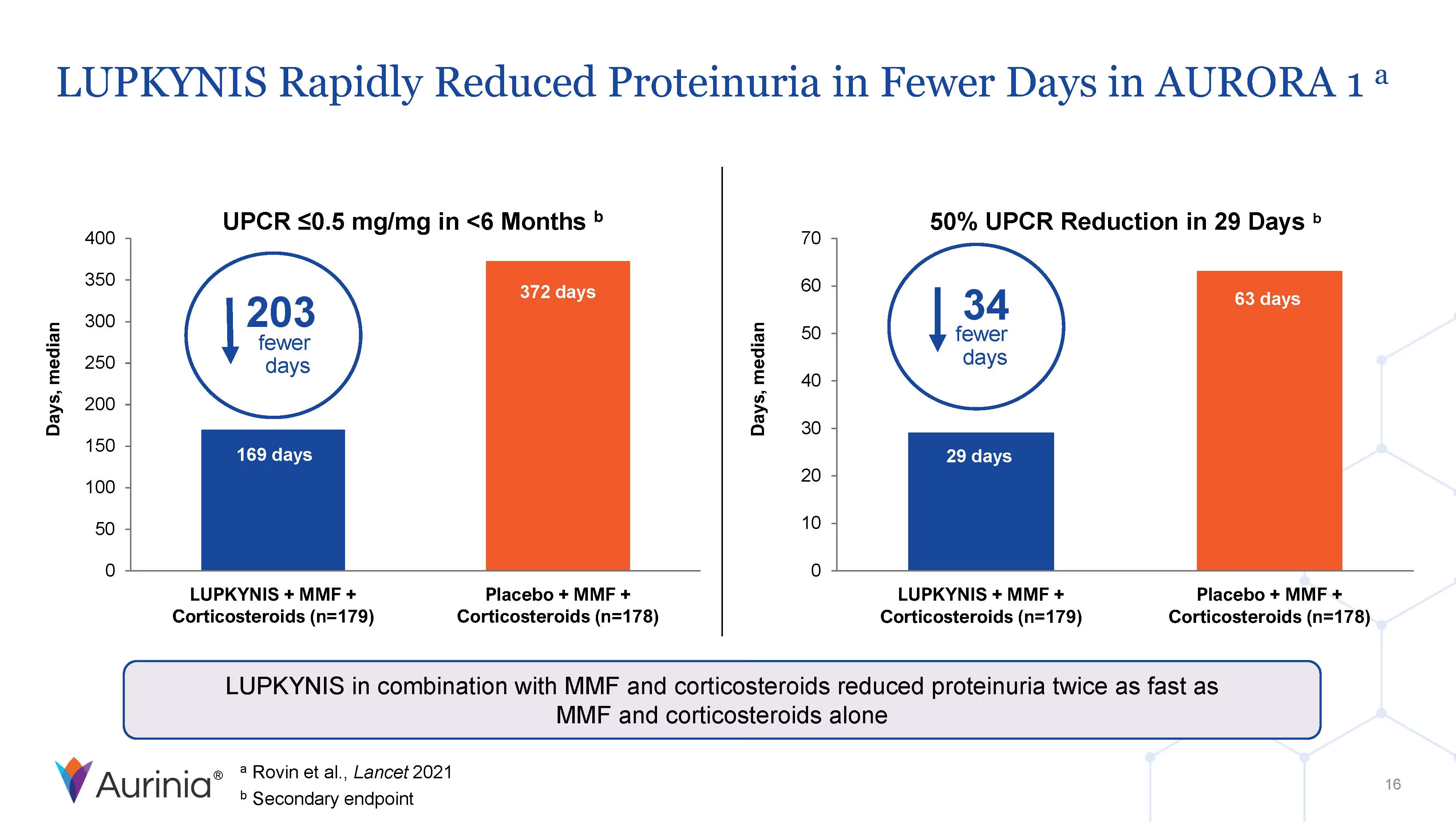
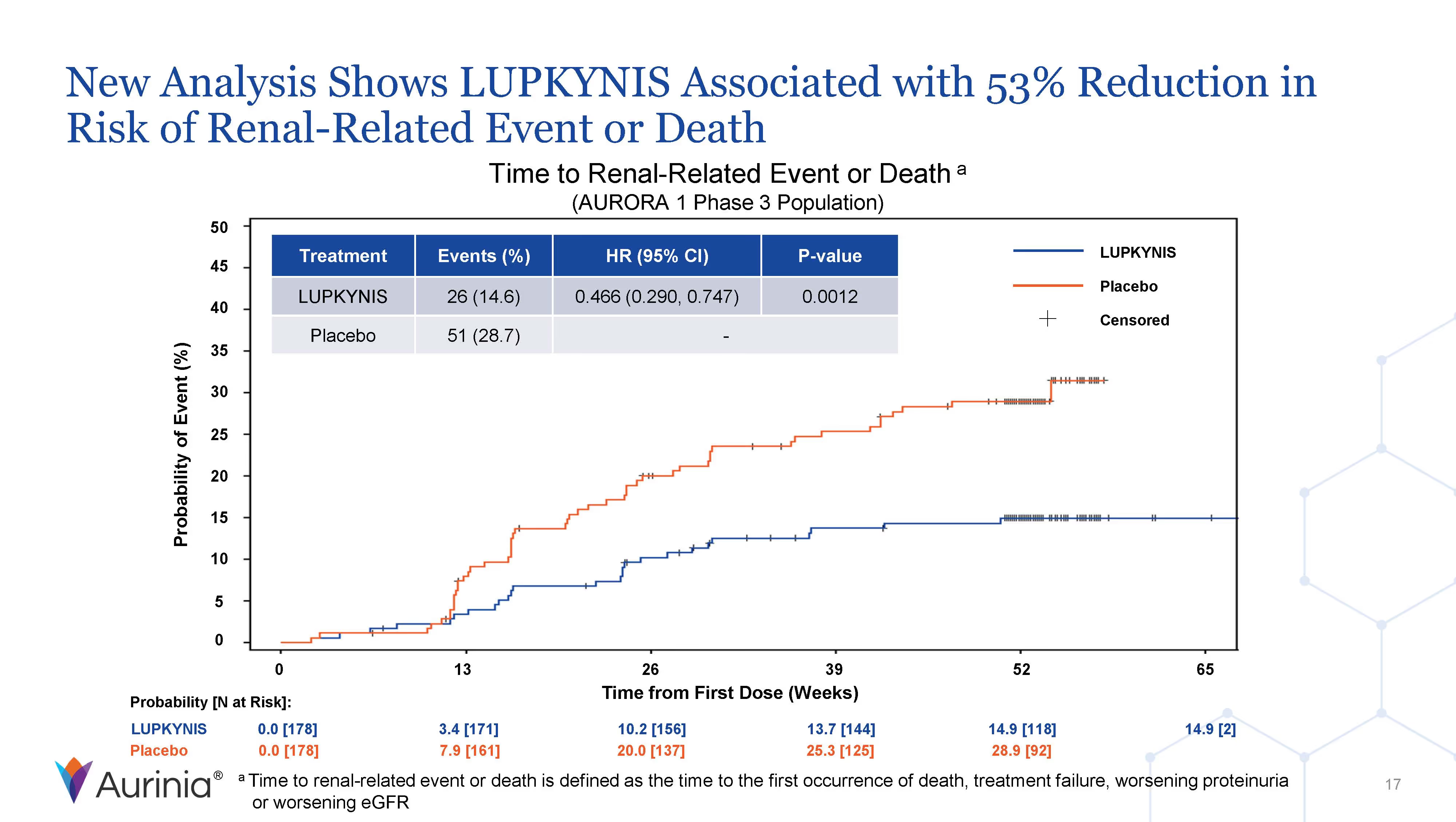
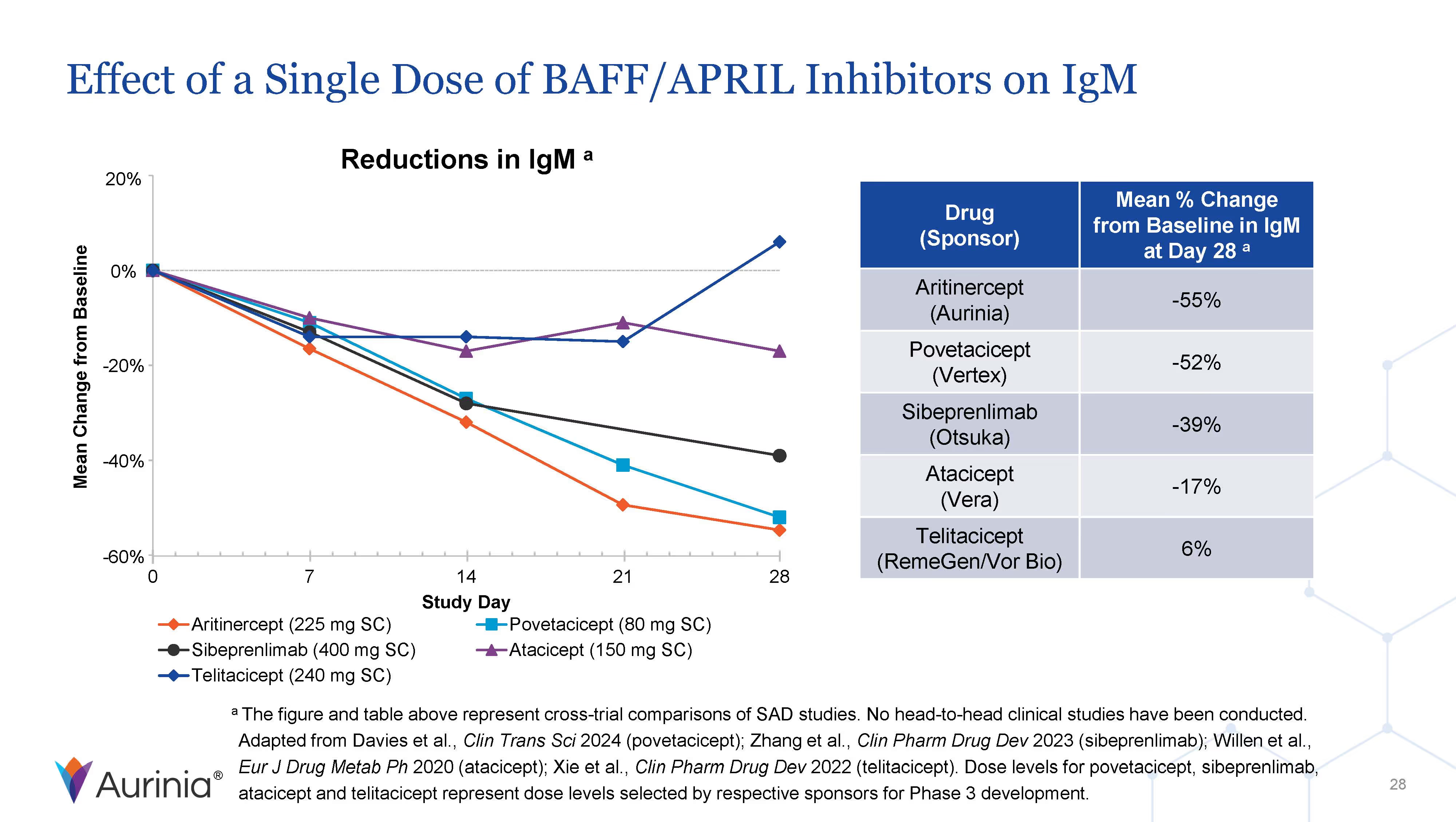
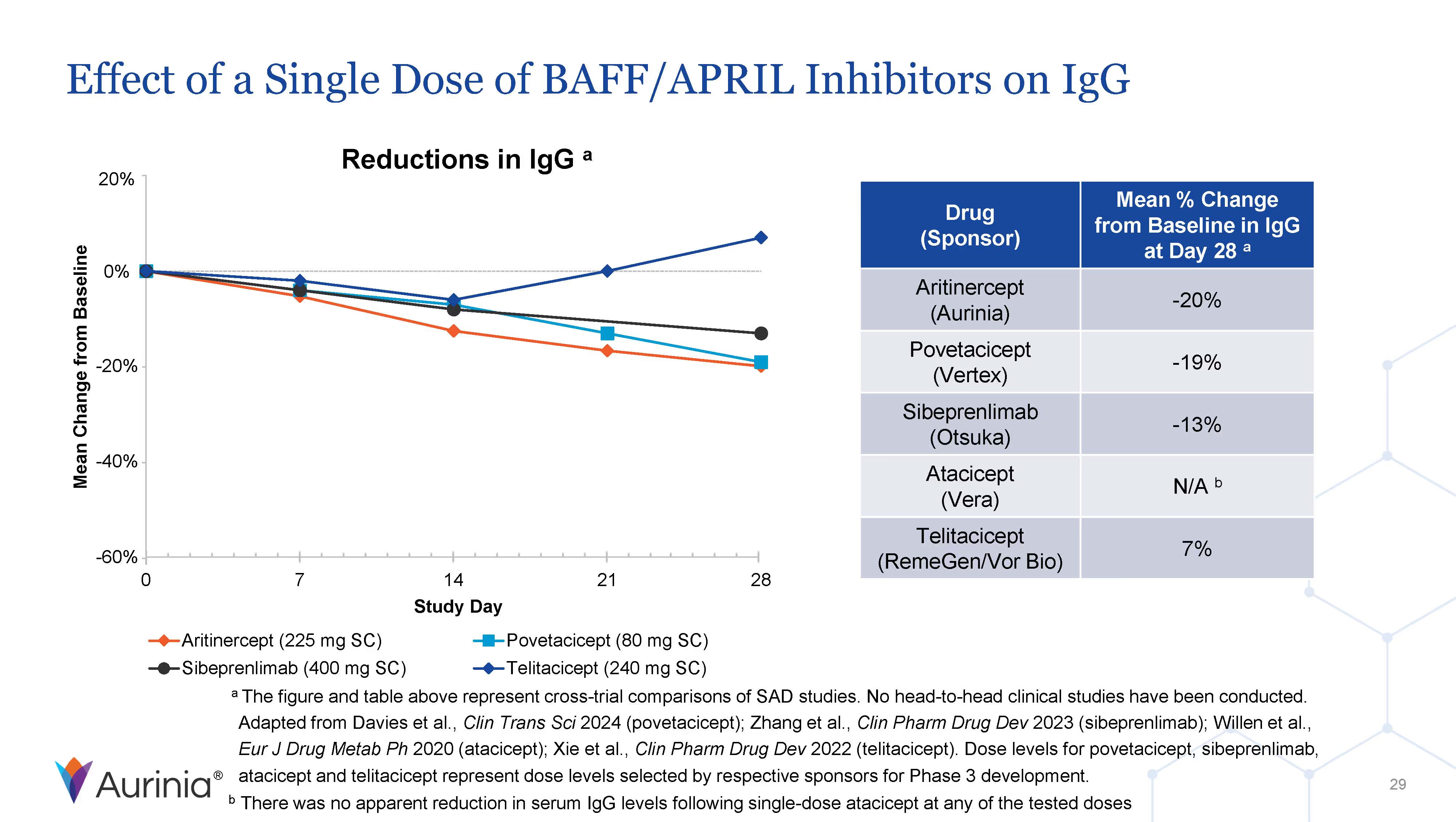
Aurinia Pharmaceuticals Inc. is a biopharmaceutical company focused on delivering therapies to people living with autoimmune diseases with high unmet medical needs. In January 2021, the Company introduced LUPKYNIS (voclosporin), the first FDA-approved oral therapy for the treatment of adult patients with active lupus nephritis. Aurinia is also developing aritinercept, a dual inhibitor of B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL) for the potential treatment of autoimmune diseases.
Email Alerts
Stock Information
NASDAQ Global Market: AUPH
-0.14
-0.95%
Volume
Upcoming Events
Past Events
American Society of Nephrology (ASN) Kidney Week 2024
2024 Cantor Fitzgerald Global Healthcare Conference
H.C. Wainwright 26th Annual Global Investment Conference
Second Quarter 2024 Financial Results Conference Call
3rd Annual H.C. Wainwright Virtual Kidney Conference
SEC Filings
Governance
IR Contact
General inquiries can be sent to ir@auriniapharma.com